의료 기기
파트너와 함께 합니다
We have been an innovator and partner to numerous successful medical projects over the past 25 years. Our patented technology enables new possibilities in the medical space, and our team has the experience and expertise to effectively integrate tactile sensing for breakthroughs in medical advancement. Whether you are looking to simply integrating our sensors for data capture, or looking for an agile, knowledgeable partner to take you from concept to creation, we’re here to help.
PPS Tactile Sensing Technology in Medical Developments
Precision Knee Aligner (PKA)
A real-time soft-tissue balancing device for total knee arthroplasty
The Precision Knee Aligner (PKA) is a surgical instrument designed to measure tibiofemoral force and compartmental load distribution during knee replacement procedures. Using PPS’s thin-film capacitive sensing technology, the PKA provides surgeons with precise, real-time feedback on ligament balance, helping to address one of the leading causes of patient dissatisfaction after surgery.
Initial development and cadaver trials have confirmed its accuracy and ease of integration into standard surgical workflows. Having recently filed a patent application, the PKA is now in the final stages of development and preparing for regulatory submission.
PKA is still in development and we are working towards an FDA approval.
맥박(脈搏)관련 개발자 키트
맥박 측정과 맥동 파형 분석
A non-invasive sensor system for capturing pulse pressure waveforms, without the need for bulky equipment like cuffs or chest straps.
The kit delivers high-fidelity pulse and waveform data for the analysis of cardiovascular signals, supporting research, algorithm development, and product testing.
It is designed as a development platform for integration into next-generation smart apparel, fitness and wellness devices, and health monitoring technologies..
성공 사례
유방 병변 진단 용(SureTouch)
FDA 승인, 방사선 없는 유방 병변 검출 시스템
SureTouch is an FDA-cleared device that uses PPS technology to detect breast masses, including breast cancer. SureTouch identifies abnormal tissue by detecting areas that are harder than the surrounding breast tissue; malignant tissue is typically much harder compared to normal breast tissue.
The key to the SureTouch device is the PPS tactile sensors in the sensor module. These sensors were developed to be ultra-sensitive and accurate.
SureTouch has since been sold and is now owned and operated by MyBexa.
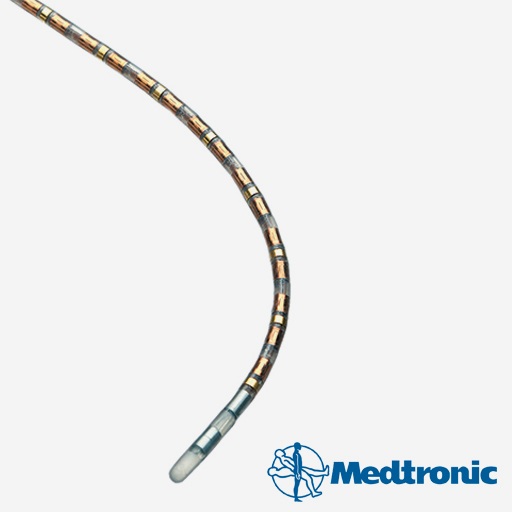
식도 상태 측정 용(ManoScan)
고분해능 식도 카테터(catheter)
The ManoScan™ ESO high-resolution manometry catheter was developed through a past PPS collaboration to enable capture of a complete physiological pressure map from the pharynx to the stomach. With 36 channels of simultaneous measurement, the system provides a detailed pressure image over time to support clinical diagnostics and research.*
*ManoScan™ and related products were sold to Medtronic. MTI/PPS is no longer involved in the manufacturing, distribution, or sale of these devices.
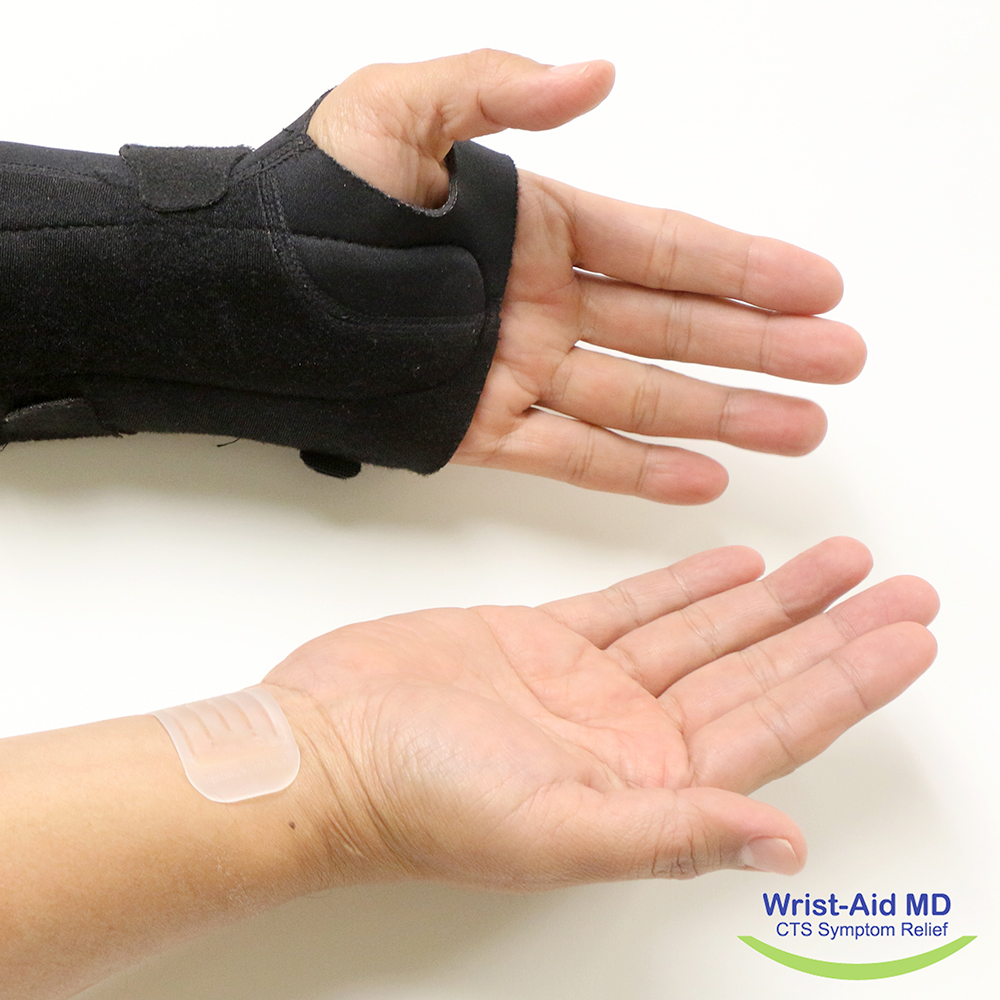
손목 부착용 통증완화(Wrist-Aid)
손목 터널 증후군 치료 장치
Wrist-Aid MD is a simple non-invasive orthotic medical device attached to the wrist and designed to alleviate carpal tunnel syndrome by applying tension to the underlying tissue to which it is attached. To better quantify these pressures and improve the design of the device, the Wrist-Aid team used a DigiTacts sensor (model 6383, 55x30mm) to capture the pressures between a patient's wrist and the device. The DigiTacts pressure sensor identified areas of increased pressure on the edges of the device and quantified the typical tension applied along the centre. Currently undergoing clinical trials, Wrist-Aid is a promising product to help alleviate symptoms of Carpal Tunnel Syndrome, which strikes millions of patients each year.
Wrist-Aid is currently on hold and not available on the market. It may be reintroduced at a later stage following further evaluation.